Multiple Choice
The following data were collected in two studies of the reaction below.A + 2B → C + D 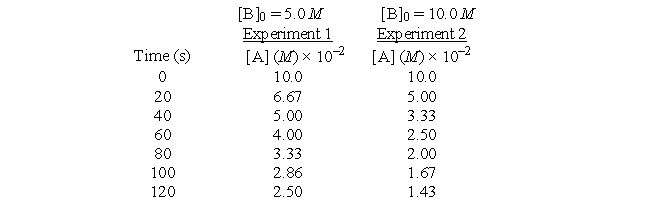
Which of the following mechanisms could be correct for this reaction?
A) A + A → E (slow)
E + B → C + D (fast)
B) A + B  E (fast)
E (fast)
E + B → C + D (slow)
C) A + B  E (fast)
E (fast)
E + A → C + D (slow)
D) none of these
Correct Answer:

Verified
Correct Answer:
Verified
Q77: Two isomers (A and B) of a
Q78: Consider the reaction<br>X<sub>2</sub>Y(g) → 2X(g) + Y(g)<br>At
Q79: For the reaction 2N<sub>2</sub>O<sub>5</sub>(g) → 4NO<sub>2</sub>(g) +
Q80: The oxidation of Cr<sup>3+</sup> to CrO<sub>4</sub><sup>2-</sup> can
Q81: For the reaction<br>P<sub>4</sub>(g) + 5O<sub>2</sub>(g) → P<sub>4</sub>O<sub>10</sub>(s)<br>the
Q83: For the reaction<br>2A + B → products<br>the
Q84: The following data were obtained for the
Q85: For the reaction 2N<sub>2</sub>O<sub>5</sub>(g) → 4NO<sub>2</sub>(g) +
Q86: For the reaction<br>2A + B → products<br>the
Q87: The following questions refer to the hypothetical