Multiple Choice
The following questions refer to the reaction 2A2 + B2 → 2C. The mechanism below has been proposed: 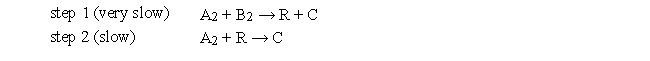
-The activation energy for the reaction H2(g) + I2(g) → 2HI(g) is changed from 184 kJ/mol to 59.0 kJ/mol at 600. K by the introduction of a Pt catalyst. Calculate the value of the ratio rate(catalyzed) /rate(uncatalyzed) .
A) 1.00
B) 0.321
C) 1.38
D) 7.62 × 1010
E) none of these
Correct Answer:

Verified
Correct Answer:
Verified
Q43: A general reaction written as 2A +
Q44: Consider the second-order reaction aA → products
Q45: The reaction A → B + C
Q46: Which quantity is greatest?<br>A) acivation energy of
Q47: For the reaction 2N<sub>2</sub>O<sub>5</sub>(g) → 4NO<sub>2</sub>(g) +
Q50: The following questions refer to the hypothetical
Q51: For the reaction<br>2A + B → products<br>the
Q52: For the reaction A + B →
Q53: The following questions refer to the reaction
Q92: How many seconds would it take for