Multiple Choice
The following questions refer to the reaction 2A2 + B2 → 2C. The mechanism below has been proposed: 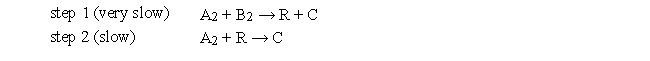
-Raw milk sours in 4.0 h at 28°C but takes 48 h to sour in a refrigerator at 5°C. Calculate the activation energy for the souring of milk.
A) 8.87 kJ
B) 4.00 kJ
C) 12.0 kJ
D) 75.2 kJ
E) none of these
Correct Answer:

Verified
Correct Answer:
Verified
Q110: A general reaction written as 2A +
Q111: The reaction<br>2NOBr → 2NO + Br<sub>2</sub><sub><br></sub>exhibits the
Q112: For a reaction a A → products,
Q113: _ is defined as the number of
Q114: The reaction<br>H<sub>2</sub>SeO<sub>3</sub>(aq) + 6I<sup>-</sup>(aq) + 4H<sup>+</sup>(aq) →
Q116: The following data were collected for the
Q117: Calculate the value of k<sub>2</sub> where<br>Rate =
Q118: The decomposition of N<sub>2</sub>O<sub>5</sub>(g) to NO<sub>2</sub>(g) and
Q119: For the reaction aA → products, select
Q120: For the reaction in the presence of