Multiple Choice
A student was trying to determine the order of a chemical reaction. To accomplish this, the student graphed the concentration - time data using various plotting methodologies. The plots are shown below. 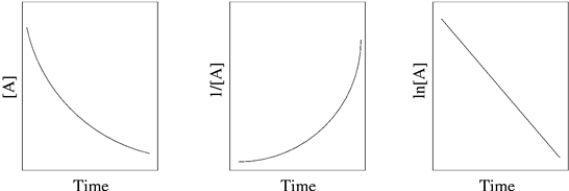 What is the order of the reaction?
What is the order of the reaction?
A) zeroth order
B) Need more data to answer the question
C) second order
D) first order
Correct Answer:

Verified
Correct Answer:
Verified
Q67: For a reaction a A → products,
Q68: For the reaction<br>2A + B → products<br>the
Q69: The following question refers to the gas-phase
Q70: The reaction<br>H<sub>2</sub>SeO<sub>3</sub>(aq) + 6I<sup>-</sup>(aq) + 4H<sup>+</sup>(aq) →
Q71: Two isomers (A and B) of a
Q73: Consider the reaction below.2NO<sub>2</sub>(g) <span
Q74: Use the potential energy diagram shown to
Q75: Which of the following statements is/are true
Q76: The following questions refer to the gas-phase
Q77: Two isomers (A and B) of a