Multiple Choice
Given the following information: 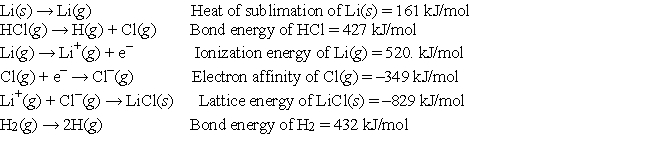 calculate the net change in energy for the reaction 2Li(s) + 2HCl(g) → 2LiCl(s) + H2(g)
calculate the net change in energy for the reaction 2Li(s) + 2HCl(g) → 2LiCl(s) + H2(g)
A) -179 kJ
B) 362 kJ
C) -70. kJ
D) -572 kJ
E) None of these
Correct Answer:

Verified
Correct Answer:
Verified
Q126: What of the following shows the bonds
Q127: Which statement is correct?<br>A) H<sub>2</sub>O is linear.<br>B)
Q128: The Lewis structure for H<sub>3</sub>BO<sub>3</sub> is<br>A) <img
Q129: In the gaseous phase, which of the
Q130: Which of the following is not a
Q132: Which of the following statements is/are true
Q133: Using the following data reactions: <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB6422/.jpg"
Q134: Of the following, which molecule has the
Q135: Select the correct molecular structure for I<sub>3</sub><sup>-</sup>.<br>A)
Q136: Use the following electronegativity values to answer