Multiple Choice
Using the following bond energies:  estimate the heat of combustion for 1 mol of acetylene:
estimate the heat of combustion for 1 mol of acetylene: 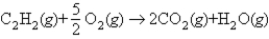
A) +365 kJ
B) 1228 kJ
C) -447 kJ
D) -1228 kJ
E) +447 kJ
Correct Answer:

Verified
Correct Answer:
Verified
Q93: Which of the following molecules has a
Q94: As indicated by Lewis structures, which of
Q95: Which of the following series is isoelectronic?<br>A)
Q96: How many Lewis structures does CO<sub>3</sub><sup>2-</sup> have?<br>A)
Q97: Consider the following molecules.<br>I. BF<sub>3</sub><br>II. CHBr<sub>3</sub> (C
Q99: Select the correct molecular structure for XeCl<sub>4</sub>.<br>A)
Q100: Which of the following statements is incorrect?<br>A)
Q101: Which of the following is polar?<br>A) PBr<sub>3</sub><br>B)
Q102: Select the correct molecular structure for PF<sub>3</sub>.<br>A)
Q103: As indicated by Lewis structures, which of