Multiple Choice
Estimate the bond energy of the N2 molecule. 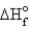 for NH3 = -46.0 kJ/mol
for NH3 = -46.0 kJ/mol
N-H bond energy = 391 kJ/mol
H-H bond energy = 432 kJ/mol
A) 1140 kJ/mol
B) 560 kJ/mol
C) 87 kJ/mol
D) 479 kJ/mol
E) 958 kJ/mol
Correct Answer:

Verified
Correct Answer:
Verified
Q44: Choose the statement that best describes the
Q45: Choose the molecule with the strongest bond.<br>A)
Q46: For each of the following compounds:<br>A) Give
Q47: How many lone pairs of electrons are
Q48: Choose the molecule with the strongest bond.<br>A)
Q50: Select the correct molecular structure for IF<sub>6</sub><sup>+</sup>.<br>A)
Q51: Which of the following has the Lewis
Q52: For which of the following can we
Q53: Consider the following molecules.<br>I. BF<sub>3</sub><br>II. CHBr<sub>3</sub> (C
Q54: Which of the following molecules has a