Multiple Choice
When molten sulfur reacts with chlorine gas, a vile-smelling orange liquid forms that is found to have the empirical formula SCl. Which of the following could be the correct Lewis structure for this compound?
A) 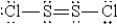
B) 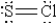
C) 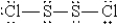
D) 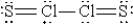
E) 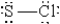
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q104: Which of the following molecules exhibits the
Q105: Which molecule or ion violates the octet
Q106: Which element listed below has the highest
Q107: How many acceptable and equivalent resonance structures
Q108: The distance between the centers of the
Q110: For each of the following compounds:<br>A) Give
Q111: Is the molecule polar or nonpolar?
Q112: Which compound does not contain both polar
Q113: Which of the following has the smallest
Q114: What does X represent in the Lewis