Multiple Choice
Complete the Lewis structure for the molecule 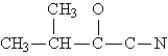 This molecule has __________ single bonds and __________ multiple bonds.
This molecule has __________ single bonds and __________ multiple bonds.
A) 11, 2
B) 6, 3
C) 13, 0
D) 11, 5
E) 4, 2
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q113: Which of the following has the smallest
Q114: What does X represent in the Lewis
Q115: Which of the following has the largest
Q116: The _ is defined as the change
Q117: _, the study of the interactions of
Q119: What type of structure does the XeOF<sub>2</sub>
Q120: Calculate the lattice energy for LiF(s) given
Q121: The electron pair in a C-F bond
Q122: How many resonance structures does the molecule
Q123: Refer to the SeF<sub>4</sub> molecule.<br>-What are the