Short Answer
Consider the following sets of quantum numbers. Which set(s) represent(s) impossible combinations? 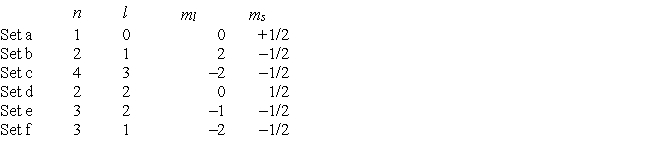
Correct Answer:

Verified
Sets b, d, and f rep...View Answer
Unlock this answer now
Get Access to more Verified Answers free of charge
Correct Answer:
Verified
Sets b, d, and f rep...
View Answer
Unlock this answer now
Get Access to more Verified Answers free of charge
Related Questions
Q46: An element E has the electron configuration
Q47: Write the electron configuration for the following:<br>-Na
Q48: How many f orbitals have the value
Q49: From the following list of observations, choose
Q50: From the following list of observations, choose
Q52: Nitrogen has 5 valence electrons. Consider the
Q53: How many unpaired electrons does arsenic have
Q54: What is the electron configuration of the
Q55: What is the valence electron configuration of
Q56: How many unpaired electrons does cobalt have