Multiple Choice
Which of the following result(s) in an increase in the entropy of the system? 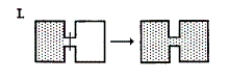 I. (See diagram.)
I. (See diagram.)
II. Br2(g) → Br2(l)
III. NaBr(s) → Na+(aq) + Br-(aq)
IV. O2(298 K) → O2(373 K)
V. NH3(1 atm, 298 K) → NH3(3 atm, 298 K)
A) I, III, IV
B) I, II, III, IV
C) I
D) II, V
E) I, II, III, V
Correct Answer:

Verified
Correct Answer:
Verified
Q9: In an isothermal process, the pressure on
Q10: Calculate ΔG for the vaporization of Br<sub>2</sub>(l)
Q11: When a gas is expanded isothermally and
Q12: Which statement is true?<br>A) There is always
Q13: 1.8 mol of an ideal gas at
Q15: A machine employs the isothermal expansion of
Q16: In which of the following changes is
Q17: Calculate w and ΔE when 1 mol
Q18: ΔS is _ for exothermic reactions and
Q19: At constant pressure, the reaction<br>2NO<sub>2</sub>(g) → N<sub>2</sub>O<sub>4</sub>(g)<br>Is