Multiple Choice
Elemental sulfur exists in two crystalline forms, rhombic and monoclinic. From the following data, calculate the temperature at which monoclinic sulfur and rhombic sulfur are in equilibrium. 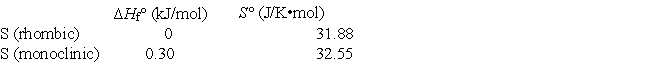
A) 0 K
B) +450 K
C) +210 K
D) -210 K
E) -450 K
Correct Answer:

Verified
Correct Answer:
Verified
Q102: The following reaction has a ΔG° value
Q103: One mole of an ideal gas is
Q104: Consider the following system at equilibrium at
Q105: One mole of an ideal gas is
Q106: ΔS<sub>surr</sub> is _ for exothermic reactions and
Q108: Which of the following is true?<br>A) For
Q109: Consider the following system at equilibrium at
Q110: Consider the process A(l) <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB6422/.jpg" alt="Consider
Q111: One mole of an ideal gas is
Q112: For which of the following processes would