Multiple Choice
Consider the following hypothetical reaction at 330 K. Standard free energies of formation are given in parentheses. 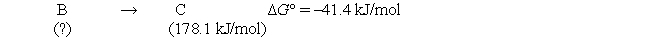 Calculate the standard free energy of formation of compound B.
Calculate the standard free energy of formation of compound B.
A) 136.7 kJ/mol
B) -314.8 kJ/mol
C) 219.5 kJ/mol
D) -219.5 kJ/mol
E) -136.7 kJ/mol
Correct Answer:

Verified
Correct Answer:
Verified
Q15: A machine employs the isothermal expansion of
Q16: In which of the following changes is
Q17: Calculate w and ΔE when 1 mol
Q18: ΔS is _ for exothermic reactions and
Q19: At constant pressure, the reaction<br>2NO<sub>2</sub>(g) → N<sub>2</sub>O<sub>4</sub>(g)<br>Is
Q21: For the reaction Cl<sub>2</sub>O(g) + (3/2)O<sub>2</sub>(g) →
Q22: At 1 atm, a liquid is heated
Q23: Consider 8.0 moles of a monatomic ideal
Q24: Calculate ΔG for the isothermal compression of
Q25: What will be the effect on the