Multiple Choice
Given the following free energies of formation: 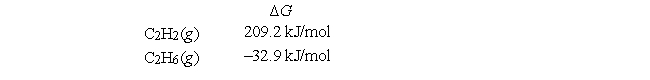 calculate Kp at 298 K for C2H2(g) + 2H2(g)
calculate Kp at 298 K for C2H2(g) + 2H2(g)  C2H6(g) .
C2H6(g) .
A) 2.72 × 1042
B) 1.24 × 1031
C) 97.2
D) 9.07 × 10-1
E) None of these is within a factor of 10 of the correct answer.
Correct Answer:

Verified
Correct Answer:
Verified
Q95: Consider the reaction<br>2NO<sub>2</sub>(g) <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB6422/.jpg" alt="Consider the
Q96: In an isothermal process, the pressure on
Q97: Consider the reaction<br>2N<sub>2</sub>O<sub>5</sub>(g) <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB6422/.jpg" alt="Consider the
Q98: Given<br>CH<sub>3</sub>CO<sub>2</sub>H(aq) <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB6422/.jpg" alt="Given CH<sub>3</sub>CO<sub>2</sub>H(aq)
Q99: For the reaction<br>CO<sub>2</sub>(g) + 2H<sub>2</sub>O(g) → CH<sub>4</sub>(g)
Q101: Consider the reaction<br>2N<sub>2</sub>O<sub>5</sub>(g) <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB6422/.jpg" alt="Consider the
Q102: The following reaction has a ΔG° value
Q103: One mole of an ideal gas is
Q104: Consider the following system at equilibrium at
Q105: One mole of an ideal gas is