Multiple Choice
The constant that connects the entropy of a system to the number of microstates is
A) 
B) 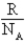
C) R
D) kB
E) B and C
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q43: Calculate the entropy change when 5.00 mol
Q44: The process H<sub>2</sub>O(g) → H<sub>2</sub>O(l) takes place
Q45: The process H<sub>2</sub>O(g) → H<sub>2</sub>O(l) takes place
Q46: Consider the process A(l) <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB6422/.jpg" alt="Consider
Q47: Given that S° = 131 J/Kmol for
Q49: In which case must a reaction be
Q50: One mole of an ideal gas is
Q51: The standard free energy of formation of
Q52: A gas expands isothermally and irreversibly.<br>-ΔG is<br>A)
Q53: At 699 K, ΔG° = -23.25 kJ