Multiple Choice
Consider the following information about the diprotic acid ascorbic acid (H2As for short, molar mass = 176.1) .  The titration curve for disodium ascorbate, Na2As, with standard HCl is shown below:
The titration curve for disodium ascorbate, Na2As, with standard HCl is shown below: 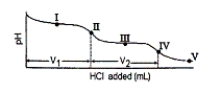
-What is the pH at point I (V1/2 HCl added) ?
A) 7.95
B) 12.39
C) 10
D) 11.79
E) none of these
Correct Answer:

Verified
Correct Answer:
Verified
Q70: Calculate the pH when 200.0 mL of
Q71: Which of the following salts shows the
Q72: You have solutions of 0.200 M HNO<sub>2</sub>
Q73: A 0.012-mol sample of Na<sub>2</sub>SO<sub>4</sub> is added
Q74: A 200.0-mL sample of the weak acid
Q76: Calculate the pH when 200.0 mL of
Q77: The contents of the flask are transferred
Q78: A 50.00-mL sample of 0.100 M Ca(NO<sub>3</sub>)<sub>2</sub>
Q79: In the titration of 100.0 mL of
Q80: One milliliter (1.00 mL) of acid taken