Multiple Choice
Consider the following information about the diprotic acid ascorbic acid (H2As for short, molar mass = 176.1) .  The titration curve for disodium ascorbate, Na2As, with standard HCl is shown below:
The titration curve for disodium ascorbate, Na2As, with standard HCl is shown below: 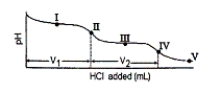
-What is the pH at point III?
A) 7.95
B) 11.79
C) 12.39
D) 4.10
E) none of these
Correct Answer:

Verified
Correct Answer:
Verified
Q130: A 200.0-mL sample of the weak acid
Q131: A solution contains 0.50 (K<sub>a</sub> = 2.0
Q132: The value of K<sub>f</sub> for the complex
Q133: Silver chromate, Ag<sub>2</sub>CrO<sub>4</sub>, has a K<sub>sp</sub> of
Q134: A student titrates an unknown weak acid,
Q136: The K<sub>sp</sub> value for PbSO<sub>4</sub>(s) is 1.3
Q137: The K<sub>sp</sub> of Al(OH)<sub>3</sub> is 2.0 ×
Q138: Consider a 100.0-mL sample of a 0.10
Q139: Calculate the solubility of Ag<sub>2</sub>SO<sub>4</sub> [K<sub>sp</sub> =
Q140: A solution contains 10. mmol of H<sub>3</sub>PO<sub>4</sub>