Multiple Choice
A 50.0-mL sample of 2.0 × 10-4 M CuNO3 is added to 50.0 mL of 4.0 M NaCN. Cu+ reacts with CN- to form the complex ion Cu(CN) 32-: 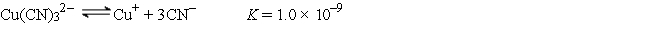 The concentration of Cu+ at equilibrium is
The concentration of Cu+ at equilibrium is
A) 5.0 × 10-14 M.
B) 1.2 × 10-14 M.
C) 2.0 × 10-4 M.
D) 1.0 × 10-4 M.
E) none of these
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q44: The concentration of Al<sup>3+</sup> in a saturated
Q45: What is the pH of this solution?<br>A)
Q46: Calculate the pH of the final solution
Q47: A 200.0-mL sample of the weak acid
Q48: Calculate the pH of a solution that
Q50: Consider a solution made by mixing 500.0
Q51: A titration of 100.0 mL of 1.00
Q52: In a solution prepared by adding excess
Q53: A 100.0-mL sample of 0.2 M (CH<sub>3</sub>)<sub>3</sub>N
Q54: After adding 25.0 mL of 0.100 M