Multiple Choice
A 50.0-mL sample of 2.0 × 10-4 M CuNO3 is added to 50.0 mL of 4.0 M NaCN. Cu+ reacts with CN- to form the complex ion Cu(CN) 32-: 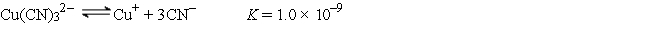 Calculate the solubility of CuBr(s) (Ksp = 1.0 × 10-5) in 1.0 L of 1.0 M NaCN.
Calculate the solubility of CuBr(s) (Ksp = 1.0 × 10-5) in 1.0 L of 1.0 M NaCN.
A) 0.33 mol/L
B) 1.0 × 10-6 mol/L
C) 1.0 × 103 mol/L
D) 1.0 mol/L
E) none of these
Correct Answer:

Verified
Correct Answer:
Verified
Q62: You are given a solution of the
Q63: A titration of 100.0 mL of 1.00
Q64: Calculate the pH when 200.0 mL of
Q65: Consider the titration of 100.0 mL of
Q66: Calculate the concentration of [H<sup>+</sup>] of a
Q68: Differentiate between the equivalence point and the
Q69: A 200-mL solution contains 0.018 mol each
Q70: Calculate the pH when 200.0 mL of
Q71: Which of the following salts shows the
Q72: You have solutions of 0.200 M HNO<sub>2</sub>