Multiple Choice
Which titration curve would result from the titration of a strong base by a strong monoprotic acid?
A) 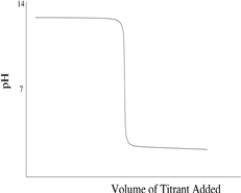
B) 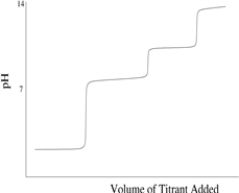
C) 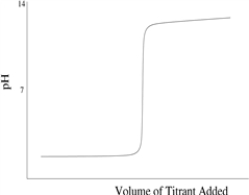
D) 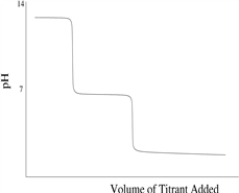
E) None of the above
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q110: Explain how the solubility of an ionic
Q111: Titrating 30.00 mL of a saturated calcium
Q112: The solubility of Cd(OH)<sub>2</sub> in water is
Q113: Explain why the pH of an aqueous
Q114: How many moles of HCl(g) must be
Q116: Calculate the solubility of Ca<sub>3</sub>(PO<sub>4</sub>)<sub>2</sub>(s) (K<sub>sp</sub> =
Q117: Which of the following statements is/are true
Q118: Calculate the pH when 200.0 mL of
Q119: The cation M<sup>2+</sup> reacts with NH<sub>3</sub> to
Q120: It is observed that 7.5 mmol of