Multiple Choice
The equilibrium constant for the reaction, Is called
A- + H+  HA
HA
A) Kb
B) 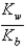
C) KwKa
D) 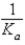
E) Ka
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q5: A 0.10-mol sample of a diprotic acid,
Q6: The salt BX, when dissolved in water,
Q7: For the equilibrium that exists in an
Q8: HCl gas is in a 1.21-L cylinder
Q9: The pH of a 0.14 M solution
Q11: Identify the Brønsted acids and bases in
Q12: Calculate [H<sup>+</sup>] in a 1.0 M solution
Q13: The following three equations represent equilibria that
Q15: Calculate the pH of the following aqueous
Q113: Explain why the pH of an aqueous