Multiple Choice
Which reaction does not proceed far to the right?
A) HCN + OH- → H2O + CN-
B) HCl + H2O → H3O+ + Cl-
C) 
D) H3O+ + OH- → 2H2O
E) 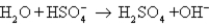
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q67: If solid sodium cyanide (NaCN) is dissolved
Q68: The following three equations represent equilibria that
Q69: HOAc K<sub>a</sub> = 1.8 ×10<sup>-5</sup><sup><br></sup>H<sub>2</sub>CO<sub>3 </sub> K<sub>a1</sub>
Q70: Calculate the pOH of a 0.10 M
Q71: Which of the following aqueous solutions will
Q73: The pH of a 0.6 M solution
Q74: Which of the following statements is true
Q75: Of the bases NaOH, H<sub>2</sub>O, CN<sup>-</sup>, SO<sub>4</sub><sup>2-</sup>,
Q76: Which of the following is the equilibrium
Q77: Calculate the pH of a 0.21 M