Multiple Choice
Determine the pH of a 7.5 M H3PO4 ( 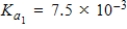 ) solution.
) solution.
A) 0.63
B) 1.02
C) 0.89
D) 0.41
E) 1.84
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q40: What is the pH in a solution
Q41: Identify the hydronium ion among the following
Q42: Calculate the pH of a 2.0 ×
Q43: The following acids are listed in order
Q44: Which of the following is a conjugate
Q46: What is the pH of a 0.2
Q47: The pH of a 0.124 M solution
Q48: Calculate the pH of the following aqueous
Q49: Calculate the pH of a 2.0 ×
Q50: The pH of a 2.1 × 10<sup>-3</sup>