Multiple Choice
Samples of the gases H2(g) and SO2(g) have equal masses and are at the same temperature and pressure. Calculate the following:
The ratio of volumes 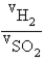
A) 32
B) 0.18
C) 180
D) 5.6
E) 1.0
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q58: Magnesium metal reacts with hydrochloric acid to
Q59: The root-mean-square velocity of a gas in
Q60: Which of the following statements is true
Q61: The valve between a 3.00-L tank containing
Q62: The ratio V/n versus the Kelvin temperature
Q64: Collision frequency between O<sub>2</sub> molecules at T<sub>1</sub>
Q65: A 0.234-g sample of a gas in
Q66: Consider two samples of helium in separate
Q67: The pressure of an ideal gas versus
Q68: A cylinder is fitted with a movable