Multiple Choice
Samples of the gases H2(g) and SO2(g) have equal masses and are at the same temperature and pressure. Calculate the following:
-The ratio of the forces per wall impact 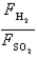 .
.
A) 5.6
B) 0.18
C) 1.0
D) 32
E) 180
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q21: Complete the following conversion:<br>1 atm = 760
Q22: Calculate the ratio of the change in
Q23: A 226.4-L cylinder contains 65.5% He(g) and
Q24: What volume of carbon dioxide measured at
Q25: Samples of the gases H<sub>2</sub>(g) and SO<sub>2</sub>(g)
Q27: It is found that 250. mL of
Q28: Consider the decomposition of C<sub>5</sub>H<sub>6</sub>O<sub>3</sub> as follows:
Q29: At STP the mass of 680.0 mL
Q30: Oxygen gas, generated by the reaction 2KClO<sub>3</sub>(s)
Q31: Consider three 1-L flasks at the same