Multiple Choice
Based on the van der Waals equation of state 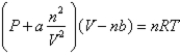 Which of the following molecules - hydrogen chloride, nitrogen dioxide, or sulfur dioxide - should behave most like an ideal gas.
Which of the following molecules - hydrogen chloride, nitrogen dioxide, or sulfur dioxide - should behave most like an ideal gas. 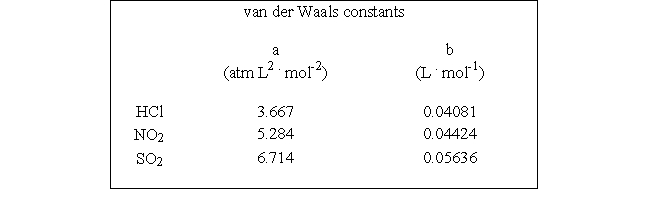
A) NO2
B) Must know P and V to answer this question
C) Must know n to answer this question
D) SO2
E) HCl
Correct Answer:

Verified
Correct Answer:
Verified
Q49: A balloon contains 10.0 g of neon
Q50: The oxidation of nitric oxide to nitrogen
Q51: The molar mass of gas Y is<br>A)
Q52: Near sea level, the atmosphere is composed
Q53: At 1000°C and 10. torr, the density
Q55: A balloon contains an anesthetic mixture of
Q56: Volume versus temperature in degrees Celsius for
Q57: How many of the following gases at
Q58: Magnesium metal reacts with hydrochloric acid to
Q59: The root-mean-square velocity of a gas in