Multiple Choice
The dehydration product of 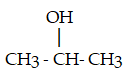 in the presence of acid is
in the presence of acid is
A) CH2 = C = CH2.
B) cyclopropane.
C) cyclopropene.
D) propene.
E) propyne.
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q5: Secondary alcohols are oxidized to _.<br>A)carboxylic acids<br>B)ketones<br>C)aldehydes<br>D)esters<br>E)ethers
Q8: Primary alcohols can be oxidized to either
Q20: The oxygen atom in alcohols decreases water
Q37: Secondary alcohols can be oxidized to ketones.
Q38: A phenol has an -OH group bonded
Q39: A secondary alcohol has a hydroxyl group
Q41: The common name for the compound CH₃
Q44: Alcohols form hydrogen bonds; this accounts for
Q45: What type of alcohol undergoes oxidation to
Q48: Diethyl ether has been replaced by halogenated