Multiple Choice
The electron states in an atom which constitute a single subshell all have:
A) only the same value of n
B) only the same value of 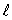
C) only the same value of n and the same value of 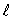
D) only the same value of 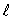 and the same value of
and the same value of 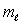
E) the same set of all four quantum numbers
Correct Answer:

Verified
Correct Answer:
Verified
Q20: Electrons in a certain laser make transitions
Q27: The minimum energy principle tells us that:<br>A)
Q28: An atom is in a state with
Q29: The states being filled from the beginning
Q33: Possible values of the principal quantum
Q35: In the relation <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB3150/.jpg" alt="
Q36: In connection with x-ray emission the
Q40: No state in an atom can be
Q60: The most energetic photon in a continuous
Q77: The Stern-Gerlach experiment makes use of:<br>A)a strong