Multiple Choice
When a lithium atom in its ground state is made from a helium atom by adding a proton (and neutron) to the nucleus and an electron outside, the electron goes into an n = 2, 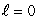 state rather than an n = 3,
state rather than an n = 3, 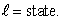 This is an indication that electrons:
This is an indication that electrons:
A) obey the Pauli exclusion principle
B) obey the minimum energy principle
C) undergo the Zeeman effect
D) are diffracted
E) and protons are interchangeable
Correct Answer:

Verified
Correct Answer:
Verified
Q2: Population inversion is important for the generation
Q3: Electrons are in a two-dimensional square potential
Q6: The electron states in an atom which
Q8: The force exerted on a magnetic dipole
Q9: The magnetic field <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB3150/.jpg" alt="
Q30: Characteristic K x-radiation of an element is
Q53: A photon with the smallest wavelength in
Q64: A laser must be pumped to achieve:<br>A)a
Q74: In a laser:<br>A)excited atoms are stimulated to
Q78: The "e" in laser stands for:<br>A)electric<br>B)emf<br>C)energy<br>D)emission<br>E)entropy