Multiple Choice
Which of the following 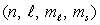 combinations is impossible for an electron in an atom?
combinations is impossible for an electron in an atom?
A) 3, 1, 1, -1/2
B) 6, 2, 0, 1/2
C) 3, 2, -2, -1/2
D) 3, 1, -2, 1/2
E) 1, 0, 0, -1/2
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q20: The K <sub> <span class="ql-formula" data-value="\infty"><span
Q21: The number of values of the orbital
Q22: Five electrons are in a two-dimensional square
Q24: An electron in an atom is
Q27: The minimum energy principle tells us that:<br>A)
Q28: An atom is in a state with
Q29: The states being filled from the beginning
Q39: Which of the following is essential for
Q40: No state in an atom can be
Q77: The Stern-Gerlach experiment makes use of:<br>A)a strong