Multiple Choice
Which of the following sets of quantum numbers is possible for an electron in a hydogen atom?
A) n = 4, 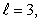
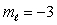
B) n = 4, 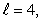
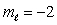
C) n = 5, 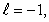
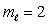
D) n = 3, 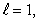
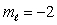
E) n = 2, 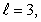
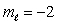
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q1: An electron is trapped in a deep
Q3: An electron in an atom initially has
Q4: Take the potential energy of a hydrogen
Q5: Take the potential energy of a hydrogen
Q7: An electron in an atom initially has
Q8: Consider the following: <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB3150/.jpg" alt="Consider the
Q9: Take the potential energy of a hydrogen
Q10: A particle is trapped in an infinite
Q11: The principle of complementarity is due to:<br>A)
Q17: The ground state energy of an electron