Multiple Choice
An ideal gas is to taken reversibly from state i, at temperature T1, to another states labeled I, II, III, IV and V on the p-V diargram below. All are at the same temperature T2. Rank the five processes according to the change in entropy of the gas, least to greatest. 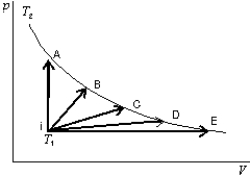
A) I, II, III, IV, V
B) V, IV, III, II, I
C) I, then II, III, Iv, and V tied
D) I, II, III, and IV, tied, then V
E) I and V tied, then II, III, IV
Correct Answer:

Verified
Correct Answer:
Verified
Q22: The temperature of n moles of a
Q27: A Carnot refrigerater runs between a
Q31: A reversible refrigerator operates between a low
Q32: The temperature T<sub>C</sub> of the cold reservoirs
Q33: Let k be the Boltzmann constant.If
Q33: A perfectly reversible heat pump with
Q38: An ideal gas, consisting of n moles,
Q50: An inventor claims to have a heat
Q55: An inventor suggests that a house might
Q59: For all irreversible processes involving a system