Multiple Choice
The pressure of an ideal gas is doubled in an isothermal process. The root-mean-square speed of the molecules:
A) does not change
B) 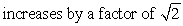
C) 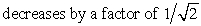
D) increases by a factor of 2
E) decreases by a factor of 1/2
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q4: The mean free path of molecules in
Q13: Air is pumped into a bicycle tire
Q16: A sample of argon gas (molar mass
Q18: A given mass of gas is enclosed
Q18: An ideal gas of N monatomic molecules
Q19: In a system of N gas molecules,
Q25: When work W is done on an
Q105: An air bubble doubles in volume as
Q106: The temperature of n moles of
Q109: According to the kinetic theory of gases,