Multiple Choice
The diagram shows the energy levels for an electron in a certain atom. Of the transitions shown, which represents the emission of a photon with the most energy? 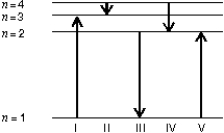
A) I
B) II
C) III
D) IV
E) V
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q3: An electron in an atom initially has
Q4: Take the potential energy of a hydrogen
Q8: An electron is in a one-dimensional trap
Q9: Take the potential energy of a hydrogen
Q11: The principle of complementarity is due to:<br>A)
Q11: The energy of a particle in a
Q14: A particle is trapped in an infinite
Q17: The ground state energy of an electron
Q24: Take the potential energy of a hydrogen
Q31: If the wave function <span