Multiple Choice
The figure shows the energy levels for an electron in a finite potential energy well. If an electron in the n = 2 state absorbs a photon of wavelength 2.0 nm, what happens to the electron? 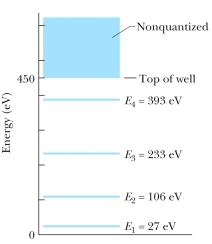
A) It makes a transition to the n = 3 state.
B) It makes a transition to the n = 4 state.
C) It escapes the well with a kinetic energy of 280 eV.
D) It escapes the well with a kinetic energy of 730 eV.
E) Nothing; this photon does not have an energy corresponding to an allowed transition so it is not absorbed.
Correct Answer:

Verified
Correct Answer:
Verified
Q7: A particle is confined to a one-dimensional
Q15: The ground state energy of an electron
Q17: The ground state energy of an electron
Q19: The series limit for the Balmer
Q19: The diagram shows the energy levels for
Q21: Take the potential energy of a hydrogen
Q34: Which of the following sets of quantum
Q35: Take the potential energy of a hydrogen
Q38: Take the potential energy of a hydrogen
Q44: When a hydrogen atom makes the transition