Multiple Choice
The following image is a dot plot of the ground state of the hydrogen atom. The dots represent: 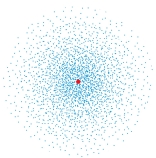
A) all the possible positions of the electron
B) every electron in the atom
C) the electron wave function
D) the volume probability density for the electron
E) the density of the electron cloud
Correct Answer:

Verified
Correct Answer:
Verified
Q6: If the wave function <img
Q8: If a wave function <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB6585/.jpg"
Q23: An electron in an atom initially has
Q30: A particle is trapped in an infinite
Q32: The figure shows the energy levels for
Q34: A particle is trapped in a one-dimensional
Q36: Consider the following: <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB3977/.jpg" alt="Consider the
Q37: The wave function for an electron in
Q39: Take the potential energy of a hydrogen
Q42: A particle is confined by finite potential