Multiple Choice
An ideal gas is to taken reversibly from state i, at temperature T1, to other states labeled I, II, III, IV and V on the p-V diagram below. All are at the same temperature T2. Rank the five processes according to the change in entropy of the gas, least to greatest. 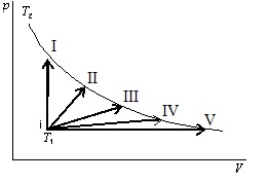
A) I, II, III, IV, V
B) V, IV, III, II, I
C) I, then II, III, IV, and V tied
D) I, II, III, and IV, tied, then V
E) I and V tied, then II, III, IV
Correct Answer:

Verified
Correct Answer:
Verified
Q3: Let S<sub>I </sub> denote the change in
Q16: Possible units of entropy are:<br>A)J<br>B)J/K<br>C)J<sup>-1</sup><br>D)liter.atm<br>E)cal/mol
Q22: The temperature of n moles of a
Q25: A heat engine absorbs energy of magnitude
Q29: Twenty-five identical molecules are in a box.Microstates
Q36: Twenty-five identical molecules are in a box.Microstates
Q37: A Carnot cycle:<br>A)is bounded by two isotherms
Q41: A hot object and a cold
Q55: An inventor suggests that a house might
Q56: A Carnot refrigerator runs between a