Multiple Choice
A sample of argon gas (molar mass 40 g) is at four times the absolute temperature of a sample of hydrogen gas (molar mass 2 g) . The ratio of the rms speed of the argon molecules to that of the hydrogen is:
A) 1
B) 5
C) 1/5
D) 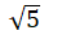
E) 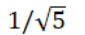
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q7: Two monatomic ideal gases are in thermal
Q13: Air is pumped into a bicycle tire
Q22: The specific heat C<sub>v</sub> at constant volume
Q28: Assume that helium behaves as an ideal
Q53: The ratio of the specific heat of
Q59: The root-mean-square speed of molecules in a
Q67: When work W is done on an
Q95: Ideal monatomic gas A is composed of
Q103: The speeds of 25 molecules are distributed
Q114: The difference between the molar specific heat