Multiple Choice
The diagram shows three isotherms for an ideal gas, with T3-T2 the same as T2-T1. It also shows five thermodynamic processes carried out on the gas. Rank the processes in order of the change in the internal energy of the gas, least to greatest. 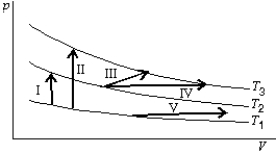
A) I, II, III, IV, V
B) V; then I, III and IV tied; then II
C) V; I; then III, and IV tied; then II
D) II; then I, III and IV tied; then V
E) II; I; then III, IV, and V tied
Correct Answer:

Verified
Correct Answer:
Verified
Q6: The internal energy of an ideal gas
Q14: Five molecules have speeds of 2.8, 3.2,
Q16: The average speed of air molecules at
Q34: The temperature of low pressure hydrogen is
Q43: Evidence that molecules of a gas are
Q45: According to the Maxwellian speed distribution, as
Q87: TV<sup> </sup> <sup> <span class="ql-formula" data-value="\gamma"><span
Q93: As the volume of an ideal gas
Q105: An air bubble doubles in volume as
Q109: According to the kinetic theory of gases,