Multiple Choice
Object A, with heat capacity CA and initially at temperature TA, is placed in thermal contact with object B, with heat capacity CB and initially at temperature TB.The combination is thermally isolated.If the heat capacities are independent of the temperature and no phase changes occur, the final temperature of both objects is:
A) (CATA - CBTB) /(CA + CB)
B) (CATA + CBTB) /(CA + CB)
C) (CATA - CBTB) /(CA - CB)
D) 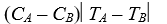
E) 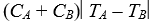
Correct Answer:

Verified
Correct Answer:
Verified
Q77: In the figure, a gas undergoes a
Q78: The diagram shows four slabs of different
Q79: In a constant-volume process with a gas,<br>A)no
Q80: The diagram shows four rectangular plates and
Q81: Take the mechanical equivalent of heat as
Q83: In an adiabatic process:<br>A)the energy absorbed as
Q84: In a certain process a gas ends
Q85: A balloon is filled with cold air
Q86: The "triple point" of a substance is
Q87: Which of the following statements pertaining to