Multiple Choice
What is the electronic geometry around the oxygen atoms in CO2? 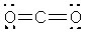
A) trigonal planar
B) linear
C) tetrahedral
D) bent
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q6: How many atoms in this molecule have
Q7: Which molecule does NOT have a lone
Q8: The Lewis structure of the cyanide ion
Q9: Which compound has an expanded octet around
Q10: Based on the Pauling electronegativities,an Fr-F bond
Q12: Which atom is the MOST electronegative?<br>A) Li<br>B)
Q13: A neutral carbon atom has _ valence
Q14: Based on the Pauling electronegativities,a C-H bond
Q15: Which atom is the MOST electronegative?<br>A) Ca<br>B)
Q16: A neutral sulfur atom has _ valence