Multiple Choice
The molecular formula for this compound is: 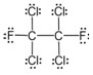
A) CFCl2
B) C2F2Cl4
C) C2(FCl) 3
D) CF2(Cl2)
Correct Answer:

Verified
Correct Answer:
Verified
Q46: Which compound is covalent?<br>A) CO<sub>2</sub><br>B) FeBr<sub>2</sub><br>C) NH<sub>4</sub>NO<sub>3</sub><br>D)
Q47: What is the CORRECT formula for hydrochloric
Q48: The systematic name for the compound K<sub>3</sub>PO<sub>4</sub>
Q49: The empirical formula for aluminum oxide is:<br>A)
Q50: Which formula is the CORRECT one for
Q52: The compound MgF<sub>2 </sub>is composed of:<br>A) polyatomic
Q53: Which compound is ionic?<br>A) SO<sub>3</sub><br>B) NF<sub>3</sub><br>C) NH<sub>3</sub><br>D)
Q54: How many valence electrons does a neutral
Q55: When two nonmetals combine,they "share" the electrons
Q56: How many valence electrons does a neutral