Multiple Choice
If the accompanying drawing of an electrochemical cell represents an iron-nickel cell,use the partial activity series included to identify the components labeled 1,2,3,and 4 respectively. 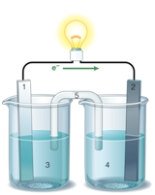 Zn Zn2+ + 2e- Fe Fe2+ + 2e-
Zn Zn2+ + 2e- Fe Fe2+ + 2e-
Co Co2+ + 2e-
Ni Ni2+ + 2e-
Cu Cu2+ + 2e-
A) iron,nickel,iron ion solution,nickel ion solution
B) nickel,iron,iron ion solution,nickel ion solution
C) nickel,iron,nickel ion solution,iron ion solution
D) iron,nickel,nickel ion solution,iron ion solution
Correct Answer:

Verified
Correct Answer:
Verified
Q37: If the accompanying drawing of an
Q38: Given the accompanying partial activity series,predict
Q39: Consider an electrochemical cell consisting of
Q40: What are the coefficients of the
Q41: Which example illustrates a redox reaction?<br>A) metal
Q43: What is the oxidation number of carbon
Q44: What is the oxidation number of sulfur
Q45: Which example represents the reduction half-reaction
Q46: Which statement is FALSE?<br>A) Silver ion (Ag<sup>+</sup>)to
Q47: Consider an electrochemical cell consisting of