Multiple Choice
If the accompanying drawing of an electrochemical cell represents an iron-nickel cell,use the partial activity series included to determine the component labeled 1. 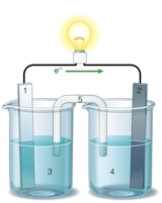 Zn Zn2+ + 2e- Fe Fe2+ + 2e-
Zn Zn2+ + 2e- Fe Fe2+ + 2e-
Co Co2+ + 2e-
Ni Ni2+ + 2e-
Cu Cu2+ + 2e-
A) iron anode
B) nickel anode
C) iron cathode
D) nickel cathode
Correct Answer:

Verified
Correct Answer:
Verified
Q6: Which example BEST represents the reaction
Q7: If the accompanying drawing of an
Q8: Knowing that sodium and potassium react violently
Q9: Which example does NOT illustrate a redox
Q10: Which reaction could be used to
Q12: The major credit for creating the first
Q13: What is the oxidation number of carbon
Q14: Given the accompanying partial activity series,which
Q15: What are the coefficients of the
Q16: Write the balanced net ionic equation