Multiple Choice
Copper has two naturally occurring isotopes with atomic masses of 62.9296 u ( 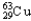 ) and 64.9278 u (
) and 64.9278 u ( 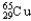 ) .The atomic mass of copper is 63.546 u.What is the percent distribution of the isotopes?
) .The atomic mass of copper is 63.546 u.What is the percent distribution of the isotopes?
A) 69.15% 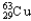 ,30.85%
,30.85% 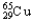
B) 30.85% 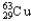 ,69.15%
,69.15% 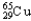
C) 1.08% 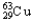 ,98.92%
,98.92% 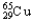
D) 98.92% 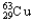 ,1.08%
,1.08% 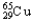
E) 99.03% 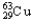 ,0.97%
,0.97% 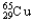
Correct Answer:

Verified
Correct Answer:
Verified
Q10: Isotopes of an element vary in mass
Q28: Which of the following statements is false?<br>A)The
Q31: Which of the following statements is false?<br>A)Atoms
Q34: Consider the following figure which represents an
Q35: Dalton's atomic theory led to a prediction
Q36: Which of the following features of Dalton's
Q39: Examine the block on the periodic table
Q40: Consider the following figure. <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB6511/.jpg" alt="Consider
Q41: The chemical symbol of germanium is Ge.An
Q45: Two natural isotopes exist for silver.51.83% of