Multiple Choice
Balance the half-reaction in acidic solution and determine the coefficient for hydrogen ion:
___ Cr3+ + ___ H2O 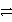
___ Cr2O72- + ___ H+
A) 2
B) 5
C) 8
D) 11
E) 14
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q3: What happens in a galvanic cell?<br>A)Cations are
Q10: What happens to an elemental oxidizing agent
Q28: Reduction can be defined as...<br>A)a increase in
Q35: Which of the following cannot be a
Q36: Balance the following redox equation in acidic
Q37: In balancing the half-reaction<br>NO(g) <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB6511/.jpg" alt="In
Q39: Which of the following is a reduction
Q41: What is the half-reaction for the oxidation
Q42: Identify the oxidation half-reaction in the redox
Q43: Balance the following redox reaction in acidic