Multiple Choice
Consider the following general wedge-and-dash diagram: 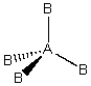 What is the molecular geometry around the central atom of this molecule?
What is the molecular geometry around the central atom of this molecule?
A) Linear
B) Tetrahedral
C) Trigonal planar
D) Bent
E) Trigonal pyramidal
Correct Answer:

Verified
Correct Answer:
Verified
Q2: Which of the following is the best
Q6: Which of the following wedge-and-dash diagrams best
Q9: Which of the following is the best
Q10: What is the molecular geometry surrounding the
Q11: Which of the following molecules is/are polar?<br>i.HF<br>ii.BH<sub>3</sub><br>iii.CBr<sub>2</sub>F<sub>2</sub><br>iv.PF<sub>3</sub><br>A)ii
Q17: What is the electron pair geometry surrounding
Q20: Which of the following statements is/are correct?<br>i.A
Q24: Which of the following has a tetrahedral
Q40: Which of the following compounds is an
Q41: Which of the following types of compounds