Multiple Choice
In a given energy level,which of the following types of orbitals will have the lowest energy?
A) 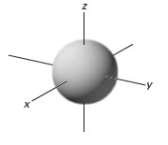
B) 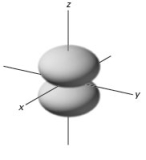
C) 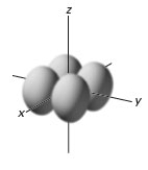
D) 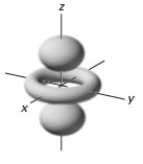
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q7: Identify the best description of an s
Q10: List the atomic numbers of all atoms
Q19: The maximum number of electrons that can
Q34: Which of the following pairs of atomic
Q36: Which of the following statements is/are incorrect?<br>i.The
Q43: Which of the following elements has the
Q45: Consider the following periodic table. <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB6511/.jpg"
Q46: Which of the following elements has the
Q48: Which of the following statements is/are correct?<br>i.Principal
Q49: Which of the following provides the best