Multiple Choice
Consider the following periodic table. 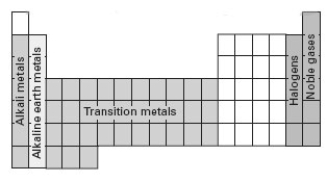 Which section represents elements whose electron configuration for the highest occupied energy level is ns2np5.
Which section represents elements whose electron configuration for the highest occupied energy level is ns2np5.
A) alkali metals
B) alkaline earth metals
C) transition metals
D) halogens
E) noble gases
Correct Answer:

Verified
Correct Answer:
Verified
Q15: Which of the following defines a correct
Q17: Consider the following periodic table. <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB6511/.jpg"
Q20: Which of the following lists electron sublevels
Q24: How many orbitals are in the 3p
Q35: Which of the following pairs of atomic
Q37: The Bohr model of the hydrogen atom
Q38: Which of the following statements is/are incorrect?<br>i.For
Q39: Which of the following correctly lists the
Q43: At any instant an electron orbital may
Q50: The order of increasing principal energy levels