Multiple Choice
Which of the following is the best definition of first ionization energy?
A) The energy required to remove one electron from a neutral gaseous atom of an element
B) The energy barrier that must be overcome to start a chemical reaction
C) The energy required to raise an electron in the ground state into the first excited state
D) The energy defined by 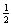 mv2
mv2
E) The energy needed to reduce a species in the aqueous phase to its minimum (first) reduction potential
Correct Answer:

Verified
Correct Answer:
Verified
Q6: Which general electron configuration is responsible for
Q8: Which of the following is the letter
Q9: Which of the following statements about atomic
Q11: How many orbitals are in the 5d
Q14: In the third principal energy level,what is
Q22: What is the ground state electron configuration
Q25: Identify the statement among the following that
Q26: Which of the following statements is/are correct?<br>i.Wavelength
Q42: Which of the following is not a
Q45: Atomic size varies with position in the